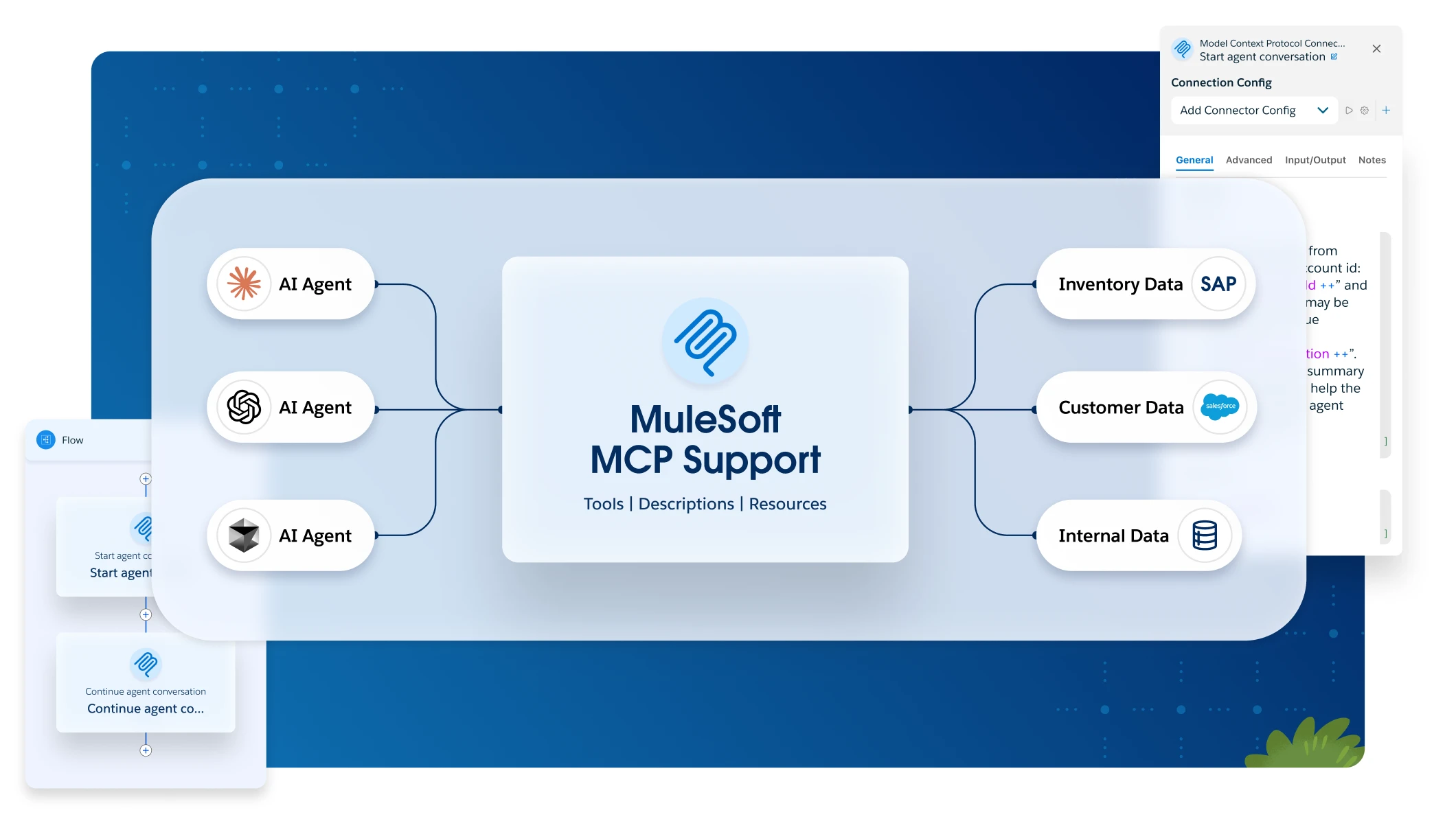
1,000+ IT leaders have weighed in. See what they are saying about AI agents in the 2026 Connectivity Benchmark Report.
Learn more.Accelerate clinical trial coordination with an API-led strategy
✓ Trust is our #1 value, so rest assured your email is safe. Learn more about the use of personal data in our Privacy Policy.
✓ Trust is our #1 value, so rest assured your email is safe. Learn more about the use of personal data in our Privacy Policy.
COVID-19 has placed a significant emphasis on the safety and efficiency of clinical trials for life-saving vaccines and therapies. However, clinical trials represent one of the most significant expenses for life sciences organizations, and most trial operations are manual and disconnected. In response, pharmaceutical companies have accelerated their adoption of digital technologies to survive a global pandemic and spur innovation in response to the expectations placed on their industry.
Join us to see how MuleSoft is partnering with the world’s leading pharmaceutical organizations, like IQVIA, to accelerate decentralized clinical trials in an unpredictable and ever-changing landscape. Learn how to:
- Automate manual processes and scale enterprise-wide automation
- Provide comprehensive, real-time insights to trial stakeholders
- Drive collaboration across various investigators and CROs
- Orchestrate a patient-centric clinical trials platform
Alexa Cushman, Director of Industry Marketing - Healthcare and Life Sciences, MuleSoft
Nagaraja Srivatsan (Sri), SVP and CDO, IQVIA
Allegra Margolis, Director of Product Marketing - Industry, MuleSoft
Vishnu Samavedula, Senior Solutions Engineer, MuleSoft