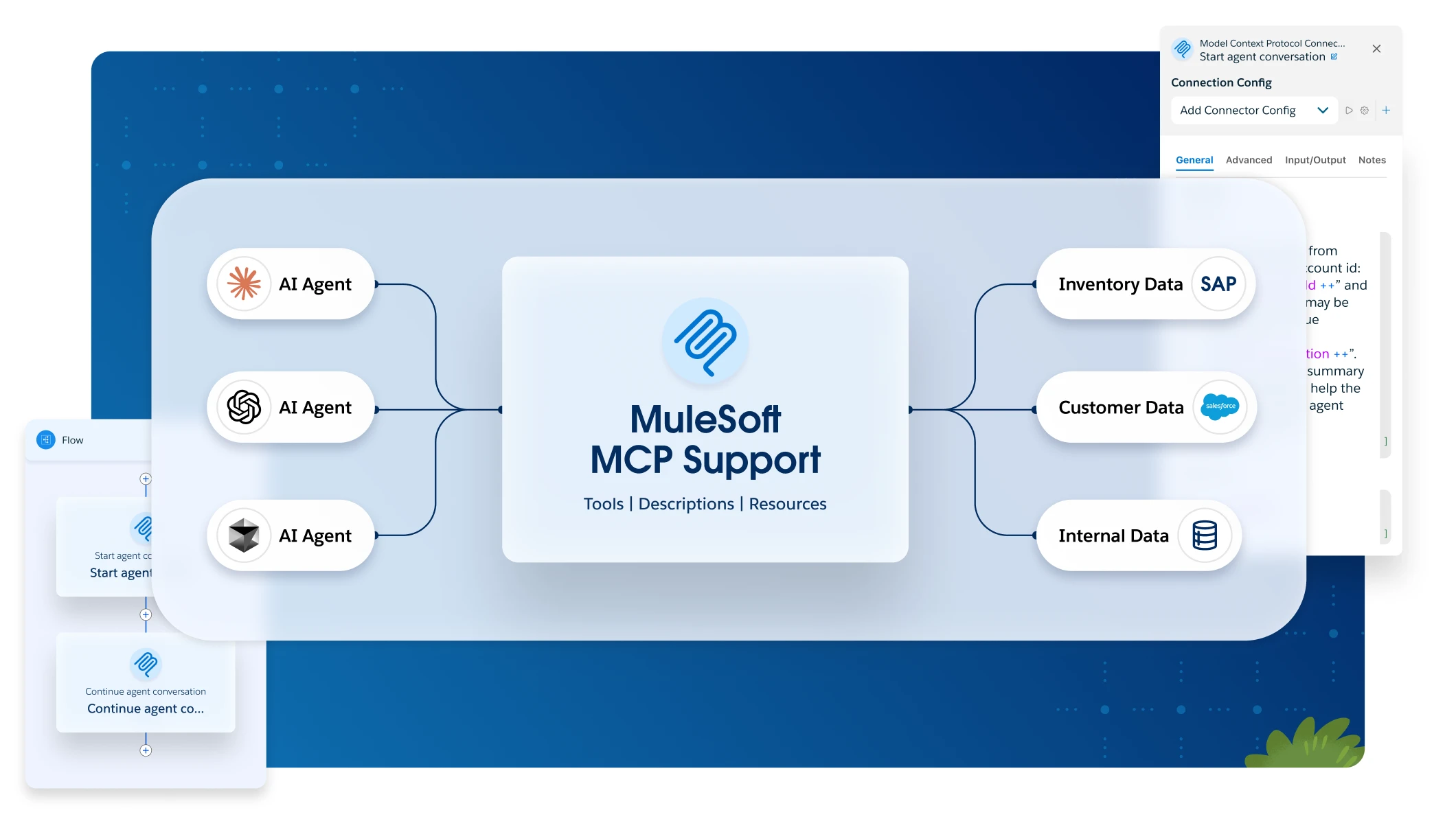
Forrester TEI Study finds 426% ROI from MuleSoft.
Read the report.Run more intelligent clinical trials
✓ Trust is our #1 value, so rest assured your email is safe. Learn more about the use of personal data in our Privacy Policy.
✓ Trust is our #1 value, so rest assured your email is safe. Learn more about the use of personal data in our Privacy Policy.
Clinical trial management is the most expensive and time-consuming segment of the research and development process. In this demo, learn how customers can surface critical study and subject data from Veeva to power visualizations — in tools like Tableau — using MuleSoft Accelerator for Life Sciences. As a result, organizations can run more efficient and intelligent clinical trials.
Ryan Stastny, Product Manager, MuleSoft